15 July 2020
The partnership with BARDA and JPEO-CBRND includes a $20 million investment for increased syringe and needle device production to support Operation Warp Speed vaccine program.
Smiths Medical, a leading global medical device manufacturer, announced today a strategic public-private partnership with Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Health and Human Services Office of the Assistant Secretary for Preparedness and Response, and the Department of Defense’s Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense (JPEO-CBRND). The partnership expands capacity at the Smiths Medical facility in Keene, New Hampshire, for production of integrated hypodermic needle and syringe products to support the government’s COVID-19 vaccination efforts.
Smiths Medical has received an order through our authorized distributor Marathon Medical’s BARDA IDIQ agreement for 78.6 million syringe and needle units. In addition, BARDA, in collaboration with JPEO-CBRND, will invest $20 million into a $38 million capital expansion project to further expand Smiths Medical’s production operations in Keene, New Hampshire. The partnership will increase Smiths Medical’s needle production capacity by 125 million units per year. The federal government will have priority access to this expanded capacity for vaccination efforts dedicated to COVID-19, flu vaccines, and future pandemics.
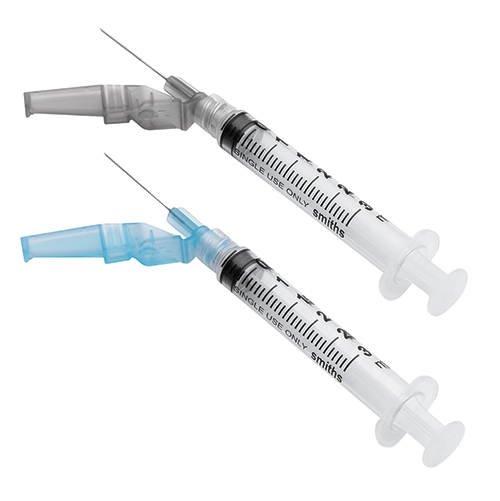
Smiths Medical’s industry-leading EDGE™ Safety Hypodermic Devices technology combines the benefits of an advanced safety hypodermic needle with the dead space profile of a conventional needle. The Needle-Pro® EDGE™ hypodermic needle offers the lowest dead space, compared to leading hypodermic safety needle devices, helping to ensure dosage accuracy and less wasted medication – while helping to prevent needlestick injuries.
“Smiths Medical has risen to the challenge of COVID-19 patient care and now delivery of a future COVID-19 vaccination. Over the last six months, Smiths Medical has delivered the healthcare industry increased production of ventilators, infusion pumps, extended dwell catheters and other respiratory products necessary for treating COVID-19 patients,” said JehanZeb Noor, Smiths Medical CEO. “The current global crisis has galvanized our culture to serve patients and transform the healthcare industry. The BARDA award is an important award in our company’s history, and we will execute relentlessly to help impact millions of lives here in the U.S.”
About Smiths Medical
Smiths Medical,www.smiths-medical.com, is a leading supplier of specialized medical devices and equipment for global markets, focusing on the medication delivery, vital care and safety devices market segments. It is part of Smiths Group, www.smiths.com, a global leader in applying advanced technologies for markets in threat and contraband detection, energy, medical devices, communications and engineered components. Smiths Group employs around 23,000 people in more than 50 countries.
General media enquiries
Contact our global media and communications team at:

Please note – the press team can only answer enquiries from accredited members of the press.
Related articles

Smiths Detection secures deal to supply systems to major cruise lines
Find out more

Smiths Interconnect expands EMEA cable harness production capacity
Smiths Interconnect, announces the expansion of its cable harness production capacity at its facility in Tunisia, an investment for growth in support of all its EMEA production sites.
Find out more

Smiths Detection accelerates AI security offering with BigBear.ai collaboration
Read our company news as Smiths Detection accelerates its AI security offering with BigBear.ai
Find out more


